spdf orbitals|s, p, d, f Atomic Orbitals : Tagatay Learn about the types, shapes, and energy levels of atomic orbitals, and how to assign quantum numbers to them. Find out how to apply Hund's rule and Pauli's exclusion principle to electron configurations. Tingnan ang higit pa CET to EST converter. Quickly convert Central European Time (CET) to Eastern Standard Time (EST) accurately using our converter and conversion table.
PH0 · s,p,d,f Orbitals
PH1 · s, p, d, f Atomic Orbitals
PH2 · What is SPDF configuration?
PH3 · SPDF orbitals Explained
PH4 · S P D F Orbitals and Angular Momentum Quantum
PH5 · Quantum Numbers
PH6 · Orbital Shapes & Quantum Numbers
PH7 · Atomic orbital
PH8 · Atomic Orbitals
PH9 · 2.4 Electron Configurations
Pèlerinage sur le Kever de Rabbi Meir Baal Haness. Rabbi Meir fait partie des plus grands Tannaïm de la quatrième génération. La seule mention de son nom est connue pour accomplir des miracles. Retrouvez l'histoire de Rabbi .
spdf orbitals*******Learn about the types, shapes, and energy levels of atomic orbitals, and how to assign quantum numbers to them. Find out how to apply Hund's rule and Pauli's exclusion principle to electron configurations. Tingnan ang higit paWhat orbitals a given atom has, and in which ones the electrons are located, depends on the energy level of the atom. Remember, . Tingnan ang higit paThe number of sublevels is given by the Angular Momentum Quantum Number – l. It takes values of 0, 1, . n-1. For example, for the second energy level, n = 2, and therefore, l . Tingnan ang higit pa
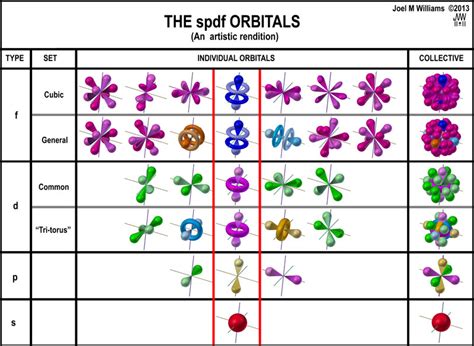
The last quantum number is the Electron Spin Quantum Number (ms) which shows the direction of the electron spin and depending on this may take a value of +1/2, represented by or -1/2, represented by ↓. Placing the direction of the arrow is important . Tingnan ang higit paThe next quantum number is the Magnetic Quantum Number, ml which shows the number of orbitals in the sublevel. It takes values . Tingnan ang higit paLearn how to represent the arrangement of electrons in orbitals of atoms using the periodic table and rules such as Pauli exclusion principle and Hund's rule. .Learn about the shapes, sizes and energies of s,p,d,f orbitals, the regions of space where electrons are most likely to be found. See examples, diagrams and explanations of how to assign electrons to orbitals in the electron configuration .
5 Answers. Sorted by: 22. s, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms. These orbitals have different shapes ( e.g. electron density distributions in .
Learn how the orbital names s, p, d, and f correspond to the angular momentum quantum number and the orbital shapes. See examples of electron configurations and the electron filling pattern in atoms. Learn about s, p, d, and f orbitals, sublevels, and their shapes with 4 quantum numbers. See how to write electron configuration, orbital notation, and orbital diagrams for .An atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. Learn about the quantum numbers, shapes, and names of orbitals, and how they are used to build the electron cloud model of atoms. This chemistry video provides a basic introduction into the quantum numbers n l ml & ms. It explains the basic idea behind the s p d f orbitals. It discuss. Learn about the shapes, energies and names of s, p, d and f orbitals in atoms. Find out how electrons are distributed in different orbitals and how they affect the chemical .
Learn how to describe the properties of an electron in an atomic orbital using quantum numbers and orbital shapes. Find out the values and meanings of , , , and , and see examples of s, p, d, and f orbitals.
Figure 2.5.6 The Three Equivalent 2p Orbitals of the Hydrogen AtomThe surfaces shown enclose 90% of the total electron probability for the 2p x, 2p y, and 2p z orbitals. Each orbital is oriented along the axis indicated by the subscript and a nodal plane that is perpendicular to that axis bisects each 2p orbital.spdf orbitals s, p, d, f Atomic Orbitals Spdf or SPDF may refer to: . Electron configuration, for which there is an obsolete system of categorizing spectral lines as "sharp", "principal", "diffuse" and "fundamental"; also the names of the sub shells or orbitals; The blocks of the periodic table, based on electron configuration as above.; Seychelles People's Defence Force, the military of SeychellesThe 2s orbital would be filled before the 2p orbital because orbitals that are lower in energy are filled first. The 2s orbital is lower in energy than the 2p orbital. There are 5 d orbitals in the d subshell. A p orbital can hold 6 .
From the abstract of Structure of the Line Spectra of the Elements as published in the 1890 Journal of the Chemical Society.. There are three kinds of series — principal, sharp (well-defined), and diffuse (ill- defined). The principal series form the most vivid lines in the spectra, and only occur in the first, periodic group ; next come the diffuse (really double) lines . Orbitals are the regions of space in which electrons are most likely to be found. > Each orbital is denoted by a number and a letter. The number denotes the energy level of the electron in the orbital. Thus 1 refers to the energy level closest to the nucleus; 2 refers to the next energy level further out, and so on. The letter refers to the shape of the orbital. The letters go . Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The number "1" represents the fact that the orbital is in the energy level closest to the nucleus. The letter "s" indicates the shape of the orbital: s orbitals are spherically symmetric around the nucleus— they look like hollow balls made of chunky material with the nucleus at . The periodic table shows us the sequential filling of the electrons .The energy of the orbitals determines the sequence of filling- Lower energy orbitals are always preferred over high energy ones.The table is thus divided into 4 blocks namely – s,p,d, f blocks, depending on the occupation of the respective orbitals by the valence electrons .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
The spdf orbital shapes are determined by the number of subshells they each have. The s-subshell has one orbital, the p-subshell has three orbitals, the d-subshell has five orbitals, and the f .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .

s orbital shape. The s orbitals are spherical; The size of the s orbitals increases with increasing shell number . E.g. the s orbital of the third quantum shell (n = 3) is bigger than the s orbital of the first quantum shell (n = 1); p orbital shape. . Atomic Orbitals are the three-dimensional space near the nucleus of an atom where the possibility of discovering an electron is maximum. The shape of an atomic orbital is associated with the quantum number and the .
spdf orbitalsOrbital Shapes – The Angular Momentum Quantum Number (l) There are four different kinds of orbitals, which are named s, p, d and f orbitals. They each have a different orbital shape. An s-orbital is spherical with the nucleus at its .Video \(\PageIndex{2}\): Looking into the probability of finding electrons. Consider the examples in Figure \(\PageIndex{3}\). The orbitals depicted are of the s type, thus l = 0 for all of them. It can be seen from the graphs of the probability densities that there are 1 – 0 – 1 = 0 places where the density is zero (nodes) for 1s (n = 1), 2 – 0 – 1 = 1 node for 2s, and 3 – 0 – 1 .Certain cookies and other technologies are essential in order to enable our Service to provide the features you have requested, such as making it possible for you to access our product and information related to your account.Geek3, Atomic-orbital-clouds spdf m0, nodes were labeled by Kathryn Haas, CC BY-SA 4.0. Exercise \(\PageIndex{2}\): Identify direction of lobes and nodes. The ability to "know" your orbitals in the context of the Cartesian coordinate system is an important skill that will help you in this course. Please draw the shapes of all of the orbitals in .s, p, d, f Atomic Orbitals Geek3, Atomic-orbital-clouds spdf m0, nodes were labeled by Kathryn Haas, CC BY-SA 4.0. Exercise \(\PageIndex{2}\): Identify direction of lobes and nodes. The ability to "know" your orbitals in the context of the Cartesian coordinate system is an important skill that will help you in this course. Please draw the shapes of all of the orbitals in .SPDF Orbitals and Sub-orbitals. Electrons exist in shells (orbitals) denoted by the principal quantum number (n = 1, 2, 3.) which corresponds to the energy state described in Bohr's model of the atom. Each shell, barring the first one, contains several sub-shells (sub-orbitals) or energy sub-levels. An electron's location is described by its .Khan Academy
The Philippines kept the top spot on the rankings of global social media usage for the sixth straight year with the average Filipino spending approximately four hours each day on social media in 2021, . Nearly half of all adult Filipinos get their news from digital sources, with 44% citing Facebook as their primary news . For example .
spdf orbitals|s, p, d, f Atomic Orbitals